Recently, research team of Professor Liu Wenguang, School of Materials Science and Engineering, Tianjin University, published a review article named “Bioinspired fabrication of high strength hydrogels from non-covalent interactions” on well-known polymer material review journal Progress in Polymer Science, with co-first author associate professor Wang Wei and Ph.D. Zhang Yinyu. The article overviewed the latest development of enhancing high strength hydrogel based on non-covalent bond, put emphasis on compacting research work of Professor Liu Wenguang in high strength hydrogel for years, summarized the importance of non-covalent bond reaction for enhancing the strength of hydrogel and the stability of water environment, explained and elaborated in detail the strengthen mechanism and application of non-covalent bond reaction to hydrogel according to the type, and finally make a generalization and expectation of future development trend of high strength hydrogel.
After hundreds of millions of years of biological evolution, organism tissues have optimized their most favorable structure for surviving and to achieve their unique functionalities. Some marine creatures, such as jellyfish and sea anemones, consist of as high as 90% water content, but their gel-like bodies exhibit exciting mechanical properties and can quickly respond to various environmental stimuli. Analogously, some human soft supporting tissues (such as tendon, ligament, meniscus, and cartilage) also resemble hydrogel substances, showing soft, tough, and impact resistance properties. A new study found that hydrophobic aggregation domains in mesoscale induced by subtle, complicated, and multiple non-covalent interactions (intra-/inter-molecule) can control the excessive swelling of these hydrogel-like tissues in water environment with high mechanical strengths. Leaning from nature, introduction of non-covalent crosslinks with proper molecular and structural designs can impart synthetic hydrogels to a suite of attractive properties: improvements in strength, toughness, resilience, processability and dynamic adaptability.
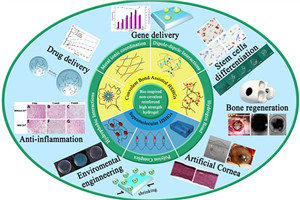
Non-covalent crosslinks, according to reaction type, generally were divided into five categories: Hydrophobic interaction, H-bonding interaction, ionic coordination, dipole-dipole interaction, and electrostatic interaction. Although the formation of non-covalent interaction is much less than of covalent bond, multiple non-covalent interactions in different types synergistically act to produce highly stable and specific associations in the polymer. When the hydrogel network loading, physical association reactions is the first breakage to dissipate mechanical energy so that it can protect the integrity of hydrogel network and have favorable mechanical loading-bearing. Benefit from the hydrophobic of non-covalent association domains, these high strength hydrogel with chemical association can remain certain content of water to avoid swelling, which makes the application of gel reactions on biological tissue substitutes prospective.
In hydrogel network system of covalent bond and dipole/h-bonding synergistic reaction, dipole/h-bonding reaction goes before covalent bonds breakage and dissipating energy. (Liu Wenguang research group. Adv Funct Mater 2015; 25: 471-80)
Utilizing the reversibility and responsiveness for environmental stimulus of these non-covalent bonds, we can design various types of stimuli sensitive shape-memory hydrogel. Because of gentle incentive condition, shape-memory hydrogel can apply to biomedical field.
Dipole enhanced ionic-driven shape memory hydrogel is to explore the influence of microgravity to stem cell differentiation.
Enhanced hydrogels based on hydrogen bonding monomer 2-vinyl-4, 6-diamino-1, 3, 5-triazine (VDT) are also reversible cell desorption. And dipole enhanced hydrogels based on mineralization can promote the skull repair of rats. (Liu Wenguang research group. Adv. Funct. Mater. 2017, 1604327)
Enhanced hydrogels base on VDT h-bonds apply on cell reverse transfection and nondestructive stripping. (Liu Wenguang research group. Adv Mater 2010; 22: 2652-6.)
The crossing points of supermolecule polymer hydrogels based on side chain double amide hydrogen bonds non covalent cross-linking can achieve dissociation and reconstitution under proper conditions, which gives gels self-repairing performance.
Supermolecule polymer hydrogels assembled by h-bonding have high-strength, high-toughness, easy processing, and self-repairing characteristics. (Liu Wenguang research group. Adv Mater 2015; 27: 3566-71)
To sum up, non-covalent bonds reactions can form mesoscopic or microscopic hydrophobic domains in hydrogel network to enhance and stabilize hydrogel network. By means of proper design, we can build shape-memory, self-repairing, hydrogel system, and easy processing hydrogels systems. Professor Liu Wenguang regarded that high-strength hydrogel research was at primary stage and the biocompatibility and mass production of hydrogels are the bottleneck of its business application. With the endless high-strength and high-toughness hydrogels, little hydrogel can reach the strength requirement of muscle tendon and other soft tissue. Perfect tissue alternative hydrogels still need further exploration. Next work mainly focuses on exploring how to widen the application of high-strength hydrogels.
The link of the article: http://www.sciencedirect.com/science/article/pii/S0079670017300047
By: the School of Materials Science and Engineering
Editors: Qin Mian and Keith Harrington






